US Food and Drug Administration Amendments Act (FDAAA) and European Medicines Agency (EMA) require that clinical trial results should be made publicly available through ClinicalTrial.gov and EU Clinical Trials Register (RU CTR). Right now, the common practice is that to save disclosure information in an XML file and then upload XML file into regulatory websites. Today, this post will make a brief introduction on two websites – ClinicalTrial.gov and EudraCT – and where to find the related guideline or documents.
ClinicalTrials.gov
ClinicalTrials.gov is a Web-based resource that provides patients, their family members, health care professionals, researchers, and the public with easy access to information on publicly and privately supported clinical studies on a wide range of diseases and conditions. The Web site is maintained by the National Library of Medicine (NLM) at the National Institutes of Health (NIH). Information on ClinicalTrials.gov is provided and updated by the sponsor or principal investigator of the clinical study. Studies are generally submitted to the Web site (that is, registered) when they begin, and the information on the site is updated throughout the study. Here takes a study as example and shows you the results of this study. You can search studies by yourself in clinicaltrials.gov.
PRS User’s Guide and Register with PRS
ClinicalTrials.gov Protocol Registration and Results System (PRS) is web-based tool used to submit clinical study information to ClinicalTrials.gov. And this webpage describes how to use the PRS and provides step-by-step instructions for PRS functions. You can find XML schema from section 9 of PRS user’s guide.
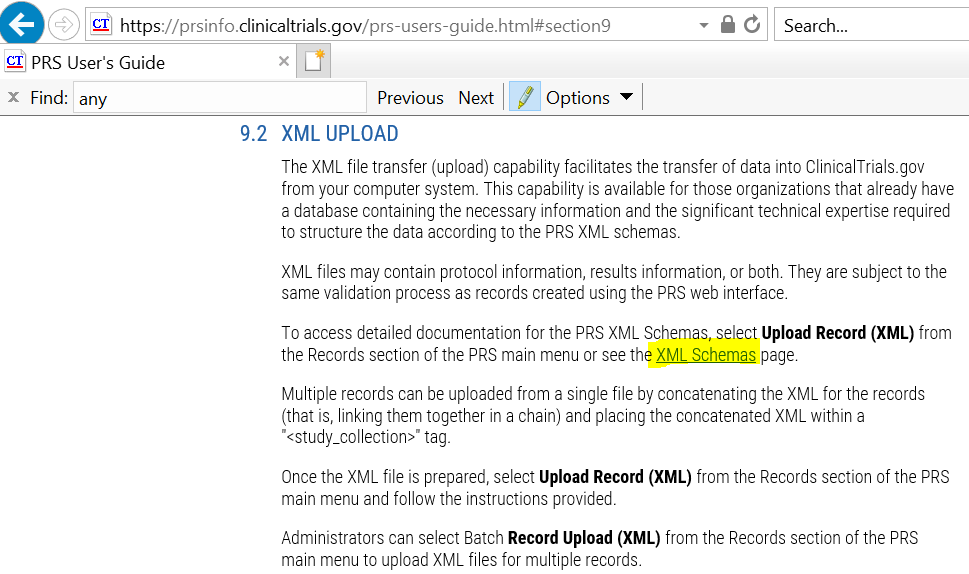 By clicking on XML Schema highlighted in yellow (from above figure), IE will direct you to following webpage and you can definitions and schema.
By clicking on XML Schema highlighted in yellow (from above figure), IE will direct you to following webpage and you can definitions and schema.
PRS Test System
PRS provides a test version of Protocol Registration and Results System (PRS). Creating and modifying records in this system will have no effect on the production PRS or ClinicalTrials.gov. However, data on this version system is occasionally replaced entirely with a copy of the latest data from the production system. You have to use the same account to login to PRS and PRS test system. And it is impossible to have an individual account without a PRS organizational account.
Training Materials for PRS
ClinicalTrials.gov staff developed online presentations to help sponsors and investigators register studies on and submit results to ClinicalTrials.gov.
EU Clinical Trials Register
The EU clinical Trials Register contains information on interventional clinical trials on medicines conducted in European Union (EU), or European Economic Area (EEA) which started after 1 May 2004. If clinical trials form part of a paediatric investigation plan or are sponsored by a marketing authorization holder, and involve the use of a medicine in the paediatric population as part of an EU marketing authorization, they should also be included even they are conducted outside the EU/EEA. Following figure shows you how to search a clinical trial from Bayer and view the results information.
EudraCT
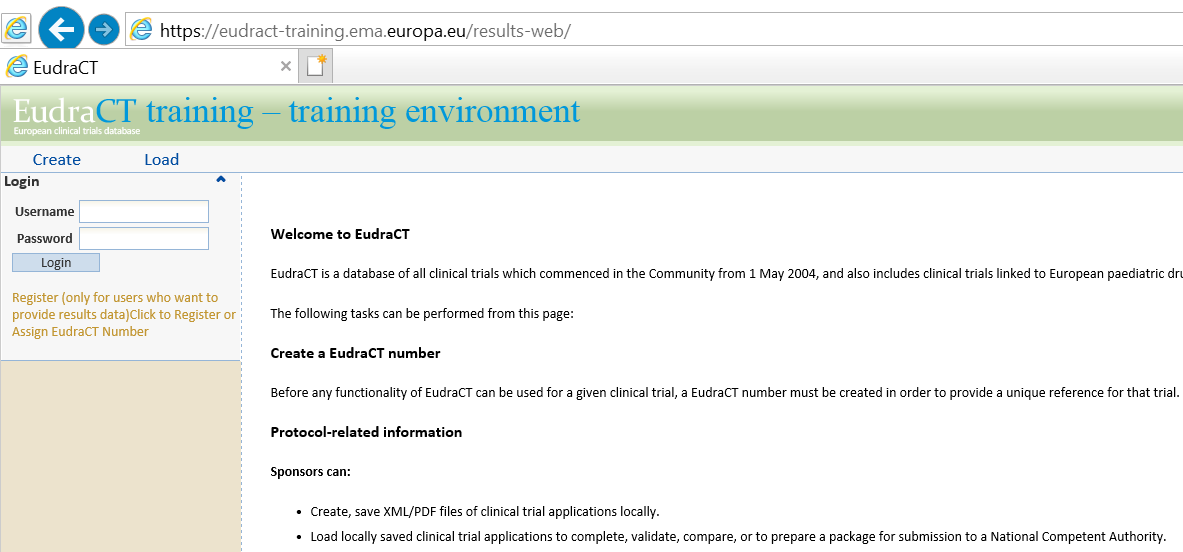
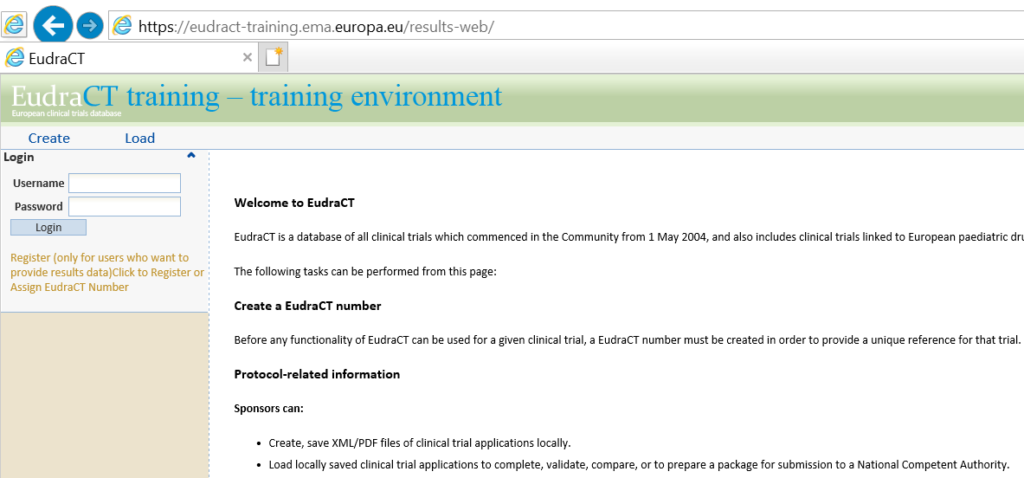
European Medicines Agency has launched a new version of European Clinical Trials Database (EudraCT). Sponsors’ representatives and can register with EudraCT and then upload XML files into EudraCT.
Open EudraCT with https://eudract.ema.europa.eu/index.html, you will see a webpage like the left part of below figure. By clicking on Results documentation, IE will direct you to the second webpage in the right side. All related documents (including XML schema) for XML creation were highlighted by red squares.
Register with EudraCT
From this webpage, you can create a EudraCT number and before any functionality of EudraCT can be used for a given clinical trial, a EudraCT number must be created.
EudraCT Result Training Environment
EduraCT result training environment can enable representatives of sponsors and sponsor-investigators to get familiarize with and get a better understanding in the preparation and posting of trials results in EudraCT.
Summary
Please note that you have to upload XML file or enter information into EudraCT or PRS. While public can view results information from EU Clinical Trials Register or ClinicalTrials.gov.
| EU | US | |
| Upload XML or Enter data | EudraCT | PRS |
| Public view results information | EU Clinical Trials Register | ClinicalTrials.gov |






Can you tell us more about this? I’d care to find out more details.
Sure.Will write new post when I aM available.