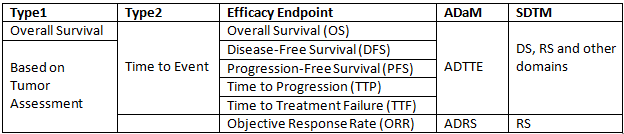
It is well known that efficacy points for oncology study are difficult to understand. This post will present details about those efficacy points and related background knowledge. Generally speaking, efficacy endpoints for oncology study can be classified into 2 categories: Overall Survival and Endpoints based on tumor assessements.
Efficacy Endpoints
Overall Survival
Overall Survival (OS) is the gold standard for demonstrating clinical benefit and often serves as primary endpoint. It is defined as the time from randomization to death from any cause and measured in the Intent-to-treat (ITT) population. It is easily and precisely to be measured. However, it requires a larger sample size and longer follow-up to show statistical difference between treatment groups. Plus, death that is not related to cancer will also be included. What’s more, it will be affected by crossover or subsequent therapies.
Endpoints Based on Tumor Assessments
There are several endpoints that are based on tumor assessments such as TTP, PFS, DFS, TTF and ORR. Related assessments, calculations and estimates are not indirect.
Disease-Free Survival (DFS)
Disease-Free Survival (DFS) is defined as the time from randomization until recurrence of tumor or death from any cause. It is frequently used as an important endpoint in adjuvant settings after definitive surgery or radiotherapy such as adjuvant breast cancer hormonal therapy, adjuvant colon cancer and adjuvant cytotoxic breast cancer therapy. Death noted without prior tumor progression documentation are usually considered as recurrences of cancer instead of censored events. This approach can minimize bias.
Objective Response Rate (ORR)
Objective Response Rate (ORR) is defined as proportion of patients with a tumor size reduction of a predefined amount. It is usually considered as sum of partial responses plus complete responses. Duration of Response (DoR) is defined as time from initial response to documented tumor progression. We can consider that ORR is a measure directly related to the drug and thus can measured in a single-arm study.ORR requires a smaller population and shorter follow-up.
Time to Progression (TTP) and Progression-Free Survival (PFS)
Progression-Free Survival (PFS) is defined as the time from randomization to disease progression or death. Time to Progression (TTP) which is defined as the time from randomizaiton to time of progression disease. PFS is the preferred regulatory endpoint when comparing with TTP. PFS assumes that deaths are randomly related to tumor progression. TTP can only be acceptable when the majority of deaths are unrelated to cancer.
They require smaller sample size and shorter follow-up. They are not affected by crossover or subsequent therapies either when comparing with Overal Survival. However, they can not be precisely measured. Frequent radiologic or other assessments is required. Moreover, timing of these assessments should be balanced among treatment groups. One last thing that I’d like to mention is that censoring methods is usually complicated.
Time to Treatment Failure (TTF)
Time to Treatment Failure (TTF) is defined as the time from randomization to treatment discontinuation for any reason, including disease progression, treatment toxicity and death. It is not recommended as a regulatory endpoint for drug approval as it can not distinguish efficacy from other variables such as toxicity. However, it is useful for studies in which drug toxicity is potentially as serious as disease progression.
Response Evaluation Criteria in Solid Tumors (RECIST)
RECIST is a set of criteria that can evaluate tumor response in oncology trials. They have been jointly developed by the European Organization for Research and Treatment of Cancer (EORTC), the National Cancer Institute (NCI) of the United States, and the National Cancer Institute of Canada Clinical Trials Group. They were initially introduced in 2000 and revised in 2009 (RECIST 1.1). This post will focus mainly on RECIST 1.1.
RECIST Terminology
In order to facilitate evaluation, tumor lesions/lymph nodes will be categorized measureable versus non-measureable and target versus non-target at baseline. The assessment method such as CT, MRI or chest X-ray can be used to characterise each identified and reported lesion at baseline and during follow-up. Target lesions are those that have been specifically measured. For Non-target lesions, their presences have been noted but measurements for them have not been taken. Here are the corresponding definitions from RECIST 1.1.
| Measurable Lesions | Non-measurable Lesions |
| Have a longest diameter of:
1) >= 10 mm by CT scan (CT scan slice thickness no greater than 5 mm) 2) >= 10 mm with calipers by clinical exam 3) >=20 mm at chest radiography 4) Malignant lymph nodes with >= 15 mm in short axis when assessed by CT scan (CT scan slice thickness recommended to be no greater than 5 mm). At baseline and in follow-up, only the short axis will be measured and followed. |
1) All other small lesions (longest diameter < 10 mm or pathological lymph nodes with >= 10 to < 15 mm short axis)
2) Truly non-measurable lesions including leptomeningeal disease, ascites, pleural or pericardial effusion, inflammatory breast disease, lymphangitic involvement of skin of lung, abdominal massess/ abdominial organomegaly |
| Target Lesions | Non-Target Lesions |
| 1) Should be measurable lesions, up to a maximum of 2 lesions per organ and 5 lesions total, representative of all involved organs, should be selected and identified as target lesions
2) Should be selected on the basis of size (those with the longest diameter) and suitability for accurate repeated measurements 3) Can only include measurable lesions |
1) Can include both measurable and non-measurable lesions
2) Measurable lesions that exceed the maximum acceptable number of target lesions 3) Non-target lesions do not need to be measured. 4) Should include all the lesions not chosen as target lesions |
Evaluation of Target Lesions (TL) versus Non-Target Lesions (NTL)
Below provides the definitions of criteria used to determine the tumor response for the group of target lesions and non-target lesions.
| Type | Evaluation Criteria | Description |
|
Target |
Complete Response (CR) | Disappearance of all target lesions.
Any pathological lymph nodes (whether target or non-target) must have reduction in short axis to <10 mm. |
| Partial Response (PR) | At least a 30% decrease in the sum of diameters of target lesions, taking as reference the baseline sum diameters. | |
| Stable Disease (SD) | Neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD, taking as reference the smallest sumdiameterswhile on study. | |
| Progressive Disease (PD) | At least a 20% increase in the sum of diameters of target lesions, taking as reference the smallest sum on study (this includes the baseline sum if that is the smallest on study). In addition to the relative increase of 20%, the sum must also demonstrate an absolute increase of at least 5 mm. (Note: the appearance of one or more new lesions is also considered progression) | |
|
Non-Target |
Complete Response (CR) | Disappearance of all non-target lesions and normalisation of tumour marker level. All lymph nodes must be non-pathological in size (<10mm short axis). |
| Non-CR/Non-PD | Persistence of one or more non-target lesion(s) and/or maintenance of tumour marker level above the normal limits. | |
| Progressive Disease (PD) | Unequivocal progression of existing non-target lesions. (Note:
the appearance of one or more new lesions is also considered progression). |
Calculation of Overall Response
For each specified time point, a response assessment should be made and overall response should be determined based on below criteria.
| Table 1 Time point response: patients with target (+/- non-target) disease | |||
| Target Lesions | Non-Target Lesions | New Lesions | Overall Response |
| CR | CR | No | CR |
| CR | Non-CR/Non-PD | No | PR |
| CR | Not evaluated | No | PR |
| PR | Non-PD or
not all evaluated |
No | PR |
| SD | Non-PD or
not all evaluated |
No | SD |
| Not all evaluated | Non-PD | No | NE |
| PD | Any | Yes or No | PD |
| Any | PD | Yes or No | PD |
| Any | Any | Yes | PD |
| Table 2 Time point response: patients with non-target disease only | ||
| Non-Target Lesions | New Lesions | Overall Response |
| CR | No | CR |
| Non-CR/Non-PD | No | Non-CR/Non-PD |
| Not all evaluated | No | NE |
| Unequivocal PD | Yes or No | PD |
| Any | Yes or No | PD |
Related ADaM domains
ADTTE is a domain which is intended for Time To Event and we should reach to ADTTE when trying to compute statistics related to Overall Survival (OS), Disease-Free Survival (DFS), Progression-Free Survival (PFS), Time to Progression (TTP), Time to Treatment Failure (TTF). As for Objective Response Rate (ORR), we have to go to ADRS.
Related SDTM domain
ADRS is simple and is derived based on SDTM RS Domain. However, ADTTE is complex and should be developed based DS, RS and even other domains such as LB according to the censor rule.
Here is a summary of this post.